This month, President Obama signed the 21st Century Cures bill into law. At nearly 1,000 pages, Cures is full of new requirements for FDA.
As I see it, some of the key provisions that biopharma, combination product, and advanced therapy companies will need to analyze and incorporate into their business strategies include:
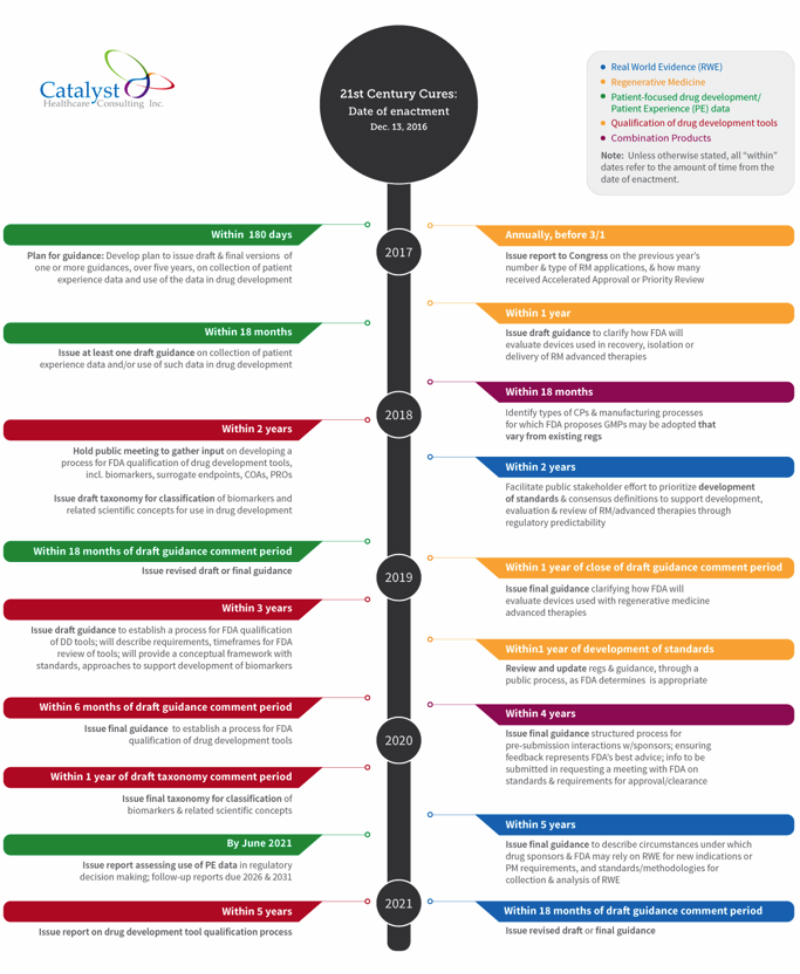
- Patient-focused drug development: FDA is required to issue methodological guidances, including for example: methodological approaches that can be taken to collect patient experience data, methods of facilitating this type of data collection in clinical trials, and methods of developing and submitting a draft guidance related to patient experience data for FDA’s consideration.
- Regenerative medicine: Establishes a definition of regenerative medicine (RM) and advanced therapies and allows FDA to consider regenerative therapies as candidates for accelerated approval.
- Real-world evidence: Requires FDA to, among other things, establish a draft framework for a program to evaluate the potential use of RWE to support approval of a new indication, or to satisfy post-market study requirements.
- Combination products: Includes several provisions intended to improve coordination among the FDA centers involved in combination product reviews and timeliness of interactions with sponsors. The law includes a section that requires new activities related to GMP requirements and pre-submission meetings with sponsors.
- Qualification of drug development tools: Requires FDA to hold a public meeting within two years to gather input on developing a process for FDA qualification of drug development tools — including biomarkers, surrogate endpoints, and clinical outcome assessments (COAs), including patient-reported outcomes (PROs)— and to issue a draft taxonomy for classification of biomarkers and related scientific concepts for use in drug development, followed by the final taxonomy within a year of the close of the draft comment period.
In this timeline, we lay out some of the specific implementation deadlines that FDA must meet under Cures over the next five years:
We explore each of these provisions further, and highlight what they mean for companies in more detail, in my recent guest column in Pharmaceutical Online.
As I note in that column, the Cures law explicitly allows the FDA Commissioner to make pay scales more competitive and improve the hiring process; thus, hopefully it will allow the agency to more easily recruit and retain top talent to help shoulder the implementation workload.